|


Anit-Freeze Who Needs It???
Applications for Anti-Freeze in Australia
As soon as someone says Anti-Freeze the immediate reaction from most people in Aus-
tralia is, who needs it. The facts are that a large number of vehicles can benefit from
anti-freeze in Australia as there is far more to Anti-Freeze than the name implies.
Anti-Freeze concentrates should really be looked at more along the lines of a complete cooling system
treatment with the added benefit of freezing protection. Tectaloy Anti-Freeze concentrate is formulated
with the best inhibitor packages and anti-foam agents available. Apart from depressing the freezing
point of a cooling system Tectaloy Anti-Freeze raises the boiling point of the system. Combine the higher
atmosphere boiling point of Anti-Freeze with the increased boiling point exerted by the radiator pressure
cap and you end up with quite substantial increases in the temperature a vehicle can run at before boiling
will take place.
Anti-Freeze concentrate also has the advantage of allowing you to mix a cooling fluid that will meet your
specific needs. Tectaloy Anti-Freeze comes with a mixing chart on the bottle so you can decide what
temperature protection level you need. Anti-Freeze concentrate is also very economical to use in some
cases where a good quality inhibitor package and freezing protection is required.
One point often overlooked is a phenomena known as wind chill factor. Wind chill is induced by moving
air, the faster it moves the colder its effect gets even though the ambient temperature is stable. The chart
below illustrates the effect of air speed on temperature.
|
Actual Air Temperature |
Wind Speed |
Effective Temperature |
0 Celsius
0 Celsius
0 Celsius
0 Celsius
0 Celsius
0 Celsius
0 Celsius
|
O0 km/h
-O0'C
08 km/h
-02' C
16 km/h
-14' C
24 km/h
-21 ./C
32 km/h
-27" C
48 km/h
-34"C
64 km/h
-37" C |
Wind speeds above 64 km/h have very little further effect on the wind chill factor.
|
As you can see from this chart, if a vehicle was parked in an area where the surrounding air temperature
was 0ø Celsius and a wind of say 24 km/h came up, blowing directly through the radiator, the propensity
for the cooling fluid to freeze quickly is drastically accelerated.
Wind chill needs to be considered in colder areas of Australia.
The Importance of Coolant pH Readings
NOTE: pH readings are no more than just an indication of how acid or alkaline (caustic)
substances are. The pH of a cooling system fluid is quite simple to read with either a small
electronic pH meter or litmus paper. Litmus paper is considered to be a little less accurate,
but will give a good indication.
pH readings go from 1 to 14, 1 being extremely acid and 14 extremely alkaline (caustic), pH 7
is considered neutral and is neither acid or alkaline.
The table below shows the pH ranges considered normal for the majority of coolants and
corrosion inhibitors running in vehicle cooling systems
|
Ph Value |
Opinion |
1
2
3
4
5
6
7
8
9
10
11.
12.
13.
14.
|
Extremely dangerous, Raw Acid. Replace coolant immediately.
Extremely dangerous, Strong Acid. Replace coolant immediately.
Extremely dangerous, Acidic. Replace coolant immediately.
Dangerous, Mild Acid. Replace coolant immediately.
Unacceptable, Mildly Acid. Replace coolant immediately.
Unacceptable, Mildly Acidic. Replace coolant ASAP
Questionable, Neutral. Probably not functioning. Replace coolant.
Acceptable.
Acceptable.
Acceptable, Alkaline. Some coolant inhibitor systems function very well at
this pH eg. GMH specification HN2043 coolant.
Unacceptable, Mildly Alkaline. Replace coolant.
Dangerous, Highly Alkaline. Replace coolant immediately.
Extremely dangerous, Highly Alkaline. Replace coolant immediately.
Extremely dangerous, Caustic. Replace coolant immediately.
|
Conclusion:
The normal running pH scope for coolants and corrosion inhibitors falls between pH 7.5 and pH 9.5.
There are inhibitor systems that can function outside of these parameters but they are in the minority.
The manufacturers of the coolants and inhibitors should be able to tell you the acceptable pH ranges
for their products.
PRESSURIZED BOILING POINTS OF GLYCOL SOLUTIONS
NOTE: The following chart gives the boiling points of Ethylene Glycol solutions under pressure.
Pressure ranges covered are from 0 - 300 kPa (0 - 45 psi). In the automotive cooling system the
pressure exerted by the radiator pressure cap usually falls between 75 - 100 kPa (11 - 15 psi).
Boiling points of all radiator fluids increase with pressure, even water has a much higher boiling
point in a pressurised radiator.
|
Pressure |
Boiling Point Celsius |
|
kPa |
psi |
33% |
55% % of Glycol |
0
25
50
75
100
150
200
250
300
|
0
3.7
7.4
11.0
14.7
22.1
26.4
29.4
44.1
|
104.5°C
110.5°C
116.5°C
121.0°C
125.0°C
132.5°C
139.0°C
146.0°C
149.5°C
|
108.5°C
114.0°C
119.5°C
125.0°C
129.0°C
136.0°C
142.5°C
150.5°C
156.5°C
|
A faulty radiator cap can cause major overheating problems in a vehicle. Always pressure check
the radiator cap for correct pressure release. If the pressure is low the vehicle will boil and 10se
radiator coolant prematurely. If the pressure release is too high the radiator or radiator hoses
can split or burst. Also check the centre vacuum valve in the cap, as the modern vehicles,
coolant recovery system will not function if this valve is faulty.
SPECIFIC GRAVITY TABLE OF GLYCOL SOLUTIONS
NOTE: Specific gravity can be used to test the approximate formulated glycol content of a vehicle's
coolingsystem. The readings below are taken at 20ø Celsius. Temperature of the fluid is important
as the specific gravity of a glycol solution falls substantially as the temperature rises.
|
Percentage of Glycol |
Specific Gravity g/cm2 |
0 0.998
10 1.012
20 1.025
30 1.038
40 1.053
50 1.065
60 1.077
70 1.088
80 1.097
90 1.105
|
As an example of the effects of increased heat on the specific gravity results we will
take the most common Glycol / Water mix used in the automotive cooling system
(approximately 30% glycol to 70% demineralized water).
At 20° C we would get an S.G. of ............1.038
At 50° C ..................................................1.023
At 70° C ..................................................1.012
At 90° C ..................................................1.002 (Approx. vehicle running temp.)
At 110° C ..: .............................................0.996
At 140° C ................................................0.972
For consistency the cootant should be tested at a known temperature when using the
specific gravity method.
FREEZING POINTS OF WATER / GLYCOL SOLUTIONS
NOTE: The freezing points given below for Ethylene Glycol solutions are actually the
point at which the first ice crystals forms, not solid freeze.
Solid freezing, the point at which the fluid will expand and cause engine damage in the
form of cracked blocks or burst radiator etc, is quite a few degrees under these freeze
points.
|
Percentage of Ethylene Glycol |
First Ice Crystal Formation Celcius |
0%
-0° C
10%
-3° C
20%
-9° C
30%
-17° C
40%
-27° C
50%
-38° C
60%
-55° C »
68%
-73° C »
»»
70%
-62° C »
80%
-45° C
90%
-27° C
99%
-13° C
|
» These ratios can give significant variation when tested.
» », This is the maximum anti-freeze protection recorded. The recording should be
considered unreliable, the general accepted standard is 60% glycol to 40% waterf0r
maximum anti-freeze protection, after this ratio the anti-freeze protection will
diminish.
BOILING UNDER PRESSURE
There are two important factors that govern the
boiling point of a vehicle. The first is the saturation
boiling point of the cooling fluid and the second is
the pressure that the fluid is under. As a very rough
guide the boiling point of a cooling system is raised
by 1 o C per 5.5kPa of radiator cap pressure. From
this calculation we can see the boiling point of a
cooling system with a 100kPa radiator cap would
be approximately 118ø C.
A fact that is often overlooked when mechanics
evaluate a cooling system is the internal running
temperatures of the cooling fluid, it is not unusual
to have coolant temperatures exceeding 140ø C in
the hotter areas of the modern engine and a general
coolant temperature in the block approaching
130ø C. This means even with a 100 kPa pressure
cap on the cooling system, water just isn't up to
the task any more and will actually be boiling
viciously in certain parts of the engine. A good
quality glycol based coolant will compensate and
|
protect the cooling system from the effects of
accelerated hot spot corrosion.
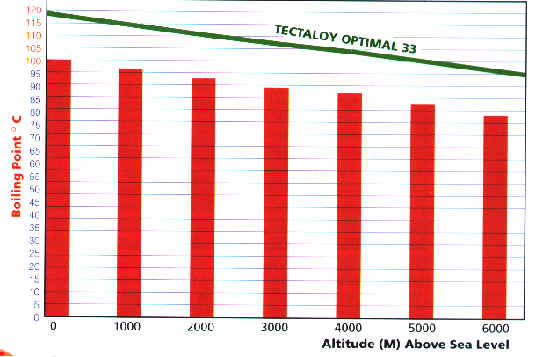
As you can see from the graph below water does
not necessarily boil at 100ø C, its boiling point is
dependant on altitude. If you increase the pressure
on water you raise it's boiling point so reducing
pressure will naturally lower the boiling point of
water. The higher above sea level you go the less
atmospheric pressure is exerted and the boiling
point is lowered.
Should the cooling system's pressurization
mechanism become faulty (the spring loaded release
valve of the radiator cap), then boiling and after-
boiling will occur more readily at higher altitudes.
The radiator cap prevents the effects of high altitude
boiling as it keeps a constant pressure on the cooling
system. A good quality glycol based coolant like
Tectaloy Optimal 33 offers additional protection
against high altitude boiling as it has a superior high
altitude boiling curve.
|
(Disclaimer: All
information, graphs, tables, and specifications are the sole property of
Tectaloy® Coolants Australia and has been taken from Tectaloy: Cooling System
Products, More than just a coolant! (vol 2) © De Ville Australia 1996)
|



